|
The Germanium Crystal
Author: Leonard Krugman

Fig. 1-2. The carbon atom in short form for transistor physics.
Like the carbon atom, the germanium atom has four valence-ring electrons. Thus a short-form illustration of the germanium atom would be similar to that shown for the carbon atom in Fig. 1-2. In addition, when germanium is in crystalline form, the four valence electrons of each atom form covalent bonds, and are tightly bound to the nucleus. Figure 1-3 (A) is a short-form illustration of the structure of the germanium crystal in this perfect state. (For simplification, the atoms in this figure are shown in a two-dimensional plane rather than in the three dimensions found in nature.) Note that all covalent bonds are complete and that no atoms or electrons are missing or misplaced. The pure germanium crystal is an insulator and is of no use in transistor work. However, pure germanium can be changed into a semiconductor by adding minute quantities of certain impurities, or by adding heat energy (phonons), or by adding light energy (photons). Any of these actions increases the number of free electrons in germanium. If an excess free electron could be added to a pure germanium crystal without changing the structure of the crystal, the electron would move through the crystal as freely as an electron moves through a vacuum tube. However, when pure germanium is treated so as to become a semiconductor, the symmetry of the crystal is destroyed. Consequently, any one excess electron moves a short distance, bounces off an imperfection, and then moves on again. This collision is similar to the collision between an electron and a gas molecule in a gas tube.
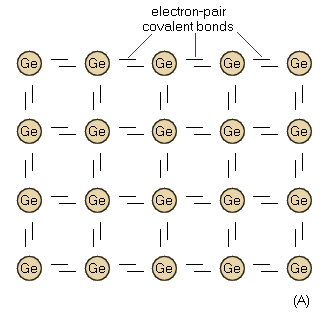
Fig. 1-3. (A) Pure germanium in crystalline form.
|


 Basic Semiconductor Physics
Basic Semiconductor Physics  Crystal Structure
Crystal Structure  The Germanium Crystal
The Germanium Crystal





 Basic Semiconductor Physics
Basic Semiconductor Physics  Crystal Structure
Crystal Structure  The Germanium Crystal
The Germanium Crystal


