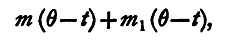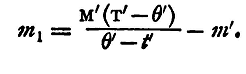| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Calorimetry Calorimetry  The Method of Mixture The Method of Mixture  Specific Heat Specific Heat |
||






|
||
Specific Heat
In this method a known mass of the material of which the specific heat is required is heated to a known temperature, and then immersed in a known mass of water also at a known temperature. A delicate thermometer is immersed in the water, and the rise of temperature produced by the hot body is thereby noted. The quantity of heat required to produce a rise of temperature of 1° in the calorimeter itself, with the stirrer and thermometer, is ascertained by a preliminary experiment. The specific heat of a substance is the ratio of the quantity of heat required to raise the temperature of a given mass of the substance 1° to the quantity of heat required to raise the temperature of an equal mass of water 1°. If we adopt as the unit of heat the quantity of heat required to raise the temperature of 1 gramme of water 1°, then it follows that the specific heat of a substance is numerically equal to the number of units of heat required to raise the temperature of 1 gramme of that substance through 1°. The mass M is cooled from T° to θ°, The quantity of heat evolved by this is therefore
assuming that the specific heat is the same throughout the range. The water in the calorimeter, the calorimeter itself, the stirrer, and the thermometer are raised from t° to θ°; the heat necessary for this is
for m1 is the heat required to raise the calorimeter, stirrer, and thermometer 1°, and the unit of heat raises 1 gramme of water 1°. But since all the heat which leaves the hot body passes into the water, calorimeter, &c., these two quantities of heat are equal. Hence
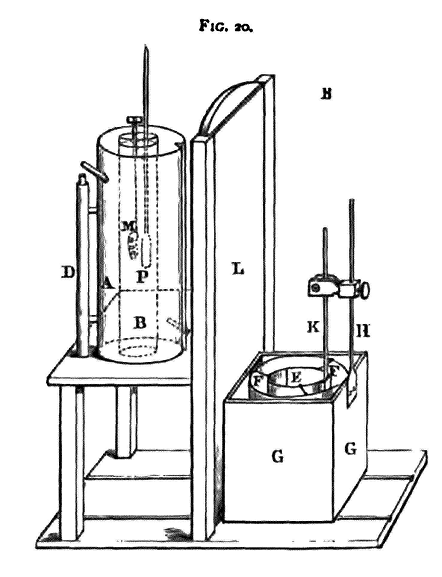
The reason for the name 'water equivalent' is now apparent, for the value found for m1 has to be added to the mass of water in the calorimeter. We may work the problem as if no heat were absorbed by the calorimeter if we suppose the quantity of water in it to be increased by m1 grammes. The quantity m1 is really the 'capacity for heat' of the calorimeter, stirrer, and thermometer. We proceed to describe the apparatus, and give the practical details of the experiments. The body to be experimented on should have considerable surface for its mass; thus, a piece of wire, or of thin sheet, rolled into a lump is a convenient form. Weigh it, and suspend it by means of a fine thread in the heater. This consists of a cylinder, A (fig. 20), of sheet copper, closed at both ends, but with an open tube, B, running down through the middle. Two small tubes pass through the outer casing of the cylinder; one is connected with the boiler, and through this steam can be sent; the other communicates with a condenser to remove the waste steam.
The cylinder can turn round a vertical axis, D, which is secured to a horizontal board, and the board doses the bottom end of the central tube. A circular hole is cut in the board, and by turning the cylinder round the axis the end of the tube can be brought over this hole. The upper end of the tube is closed with a cork, which is pierced with two holes; through the one a thermometer, P, is fixed, and through the other passes the string which holds the mass M. The thermometer bulb should be placed as close as possible to M. The steam from the boiler is now allowed to flow through the outer casing, raising the temperature of the mass M; the cylinder is placed in such a position that the lower end of the tube in which M hangs is covered by the board. The temperature in the enclosed space will rise gradually, and it will be some time before it becomes steady, After some considerable interval it will be found that the thermometer reading does not alter, the mercury remaining stationary somewhere near 100°. Note the reading; this is the value of T in the above equation (1). While waiting for the body to become heated the operation of finding the water equivalent of the calorimeter may be proceeded with. The calorimeter consists of a copper vessel, E, which is hung by silk threads inside a larger copper vessel, F. The outside of the small vessel and the inside of the large one should be polished, to reduce the loss of heat by radiation. This larger vessel is placed inside a wooden box, G, to the bottom of which slides are fixed. These slides run in grooves in the wooden baseboard of the apparatus, and the box can be pushed easily, under the board to which the heater is attached, being just small enough to slide under it. When the box is thus pushed into position the calorimeter is under the hole in the board which has already been mentioned -, and if the cylinder be turned so that its inner tube may come over this hole, the heated body can be dropped directly into the calorimeter. L is a sliding screen, which serves to protect the calorimeter from the direct radiation of the heater, and which must be raised when it is required to push the calorimeter under the heater. A brass rod, H, is attached to the back of the box G, and carries a clip in which a delicate thermometer, K, is fixed. The thermometer bulb is in the calorimeter, a horizontal section of which is a circle with a small square attached to it; the thermometer is placed in the square part, and is thus protected from injury by the mass M when it is immersed, or by the stirrer. The stirrer is a perforated disc of copper, with a vertical stem. A wooden cover with a slot in it,
through which the stirrer and thermometer pass, fits over the box G. There is a long vertical indentation in the heater A, and the upper part of the thermometer can fit into this when
Let us suppose that it has been found, either from a rough experiment or by calculation from an approximate knowledge of the specific heat of the substance, that if the calorimeter be rather more than half full of water the hot body will raise its temperature by about 4°. Then, in determining the water equivalent, we must endeavour to produce a rise in temperature of about 4°, starting from the same temperature as we intend to start from in the determination of the specific heat.
Weigh the calorimeter. Fill it rather more than half full of water, and weigh it again. Let m' be the increase in mass observed; this will be the mass of water in the calori. meter; let t' be the temperature of the water. The experiment is performed by adding hot water at a known temperature to this and observing the rise in temperature. If the hot water be poured in from a beaker or open vessel its temperature will fall considerably before it comes in contact with the water in the calorimeter. To avoid this there is provided a copper vessel with an outer jacket. The inner vessel can be filled with hot. water, and the jacket prevents it from cooling rapidly. A copper tube with a stopcock passes out from the bottom of the vessel, and is bent vertically downwards at its open end. This tube can pass through the slot in the covering of the wooden box G close down to the surface of the water in the calorimeter. A thermometer inserted in a cork in the top of the vessel serves to read the temperature of the hot water. For the present purpose this may be about 30°. It is not advisable that it should be much higher.
Turn the tap of the hot-water vessel, and let some water run into a beaker or other vessel; this brings the tube and tap to the same temperature as the water that will be used. Turn the tap off, and place the calorimeter, which should be in the wooden box, with the thermometer and stirrer in position, underneath the tube, and then turn the tap again, and allow the hot water to run into the calorimeter rather slowly. The temperature of the water in the calorimeter rises. When it has gone up about 3° stop the hot water from flowing. Stir the water in the calorimeter well; the temperature will continue to rise, probably about 1° more; note the highest point which the mercury in the thermometer attains. Let the temperature be θ'. Note the temperature of the hot water just before and just after it has been allowed to flow into the calorimeter; the two will differ very little; let the mean be T'. This may be taken as the temperature of the hot water. Weigh the calorimeter again; let the increase in mass be M' grammes. This is the mass of hot water which has been allowed to flow in, and which has been cooled from T' to θ'. The heat given out is
It has raised the temperature of the calorimeter, stirrer, &c., and a mass m' of water from t' to θ'. The heat required to do this is
and this must be equal to the heat given out by the hot water in cooling, m1 being, as before, the required water equivalent. Hence
and
In doing this part of the experiment it is important that the apparatus should be under the same conditions as when determining the specific heat. The measurements should be made, as we have said, with the calorimeter in the box, and the initial and final temperatures should be as nearly as may be the same in the two experiments. The error arising from loss by radiation will be diminished if the experiment be adjusted so that the final temperature is as much above that of the room as the initial temperature was below it.
Having found the water equivalent of the calorimeter, we proceed to determine the specific heat of the substance. The mass of the empty calorimeter is known; fill the calorimeter with water from one-half to two-thirds full; weigh it, and thus determine m, the mass of the water. Replace the calorimeter in the wooden box on the slides of the apparatus, and take the temperature of the water two or three times to see if it has become steady; the final reading will be the value of t. Note also the temperature of the thermometer P; when it is steady raise the slide L, and push the box G under the heater, turning the latter round the axis D until the tube B is over the hole in the stand. Then by loosening the string which supports it drop the mass M into the calorimeter. Draw the box back into its original position, and note the temperature with the thermometer K, keeping the water well stirred all the time, but being careful not to raise the substance out of the water. When the mercury column has risen to its greatest height and is just beginning to recede read the temperature. This gives the value of θ, the common temperature of the substance and the water.
Thus all the quantities in the equation for the specific heat have been determined, and we have only to make the substitution in order to find the value.
The same apparatus may be used to determine the specific heat of a liquid, either by putting the liquid into a very thin vessel, suspending it in the heater, and proceeding in the same way, allowing, of course, for the heat emitted by the vessel, or by using the liquid instead of water in the calorimeter, and taking for the mass M a substance of known specific heat. Thus C would be known, and if m be the mass of the liquid, c its specific heat, we should have
Hence
t, θ, and T having the same meaning as above.
Experiment. - Determine by the method of mixture the specific heat of the given substance, allowing for the heat absorbed by the calorimeter &c.
Enter results thus : -
|
||
Home  Calorimetry Calorimetry  The Method of Mixture The Method of Mixture  Specific Heat Specific Heat |
||
Last Update: 2011-03-27