| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Atoms Atoms  Quantum Energy Levels In Atoms Quantum Energy Levels In Atoms  The Photoelectric Effect The Photoelectric Effect |
|||||||






|
|||||||
The Photoelectric EffectAuthor: John Hutchinson
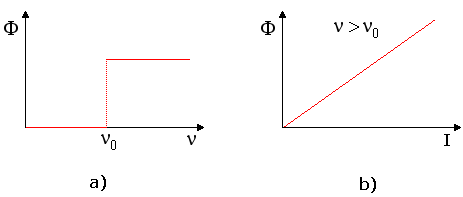
When a light source is directed at a metal surface, it is found under many circumstances that electrons are ejected from the surface. This phenomenon is called the "photoelectric effect." These electrons can be collected to produce a usable electric current. (This effect has a variety of common practical applications, for example, in "electric eye" devices.) It is reasonable to expect that a certain amount of energy is required to liberate an electron from a metal surface, since the electron is attracted to the positively charged nuclei in the metal. Thus, in order for the electron to escape, the light must supply sufficient energy to the electron to overcome this attraction. The following experimental observations are found when studying the photoelectric effect. First, in order for the effect to be observed, the light must be of at least a minimum frequency which we call the threshold frequency, ν0. This frequency is a characteristic for a given metal. That is, it is the same value for each sample of that metal, but it varies from one metal to the next. For low frequency light, photoelectrons are not observed in any number, no matter how intense the light source is. For light with frequency above ν0, the number of photoelectrons emitted by the metal (measured by the photoelectric current, Φ) increases directly with the intensity of the light. These results are shown in figure 1.
Second, we can measure the energies of the electrons emitted by the metal. For a given metal, all photoelectrons have the same kinetic energy for a fixed frequency of light above ν0. This fixed kinetic energy is independent of the intensity of the light source. As the frequency of the light is increased, the kinetic energy of the emitted electrons increases proportionally. These results are shown in figure 2.
Are these results surprising? To the physicists at the end of the nineteenth century, the answer was yes, very surprising indeed. They expected that the energy of the light source should be determined by its intensity. Hence, the energy required to eject a photoelectron should be supplied by light of high intensity, no matter how low the frequency of the radiation. Thus, there should be no threshold frequency, below which no electrons are emitted. Moreover, the kinetic energy of the electrons should increase with intensity, not with light frequency. These predictions are not observed, so the results are counter to physical intuition. We can account for these results in a straightforward but perhaps non-obvious manner. (Einstein provided the explanation in 1905.) Since the kinetic energy of the emitted photoelectrons increases proportionally with increases in the frequency of the light above the threshold frequency, we can conclude from conservation of total energy that the energy supplied by the light to the ejected electron must be proportional to its frequency. This does not immediately account for the existence of the threshold frequency, though, since it would still seem to be the case that even low frequency light would possess high energy if the intensity were sufficient. By this reasoning, high intensity, low frequency light should therefore produce as many photoelectrons as are produced by low intensity, high frequency light. But this is not observed. This is a very challenging puzzle, and an analogy helps to reveal the subtle answer. Imagine trying to knock pieces out of a wall by throwing objects at it. We discover that, no matter how many ping pong balls we throw, we cannot knock out a piece of the wall. On the other hand, only a single bowling ball is required to accomplish the task. The results of this "experiment" are similar to the observations of the photoelectric effect: very little high frequency light can accomplish what an enormous amount of low frequency light cannot. The key to understanding our imaginary experiment is knowing that, although there are many more ping-pong balls than bowling balls, it is only the impact of each individual particle with the wall which determines what happens. Reasoning from this analogy, we must conclude that the energy of the light is supplied in "bundles" or "packets" of constant energy, which we will call photons. We have already concluded that the light supplies energy to the electron which is proportional to the light frequency. Now we can say that the energy of each photon is proportional to the frequency of the light. The intensity of the light is proportional to the number of these packets. This now accounts for the threshold frequency in a straightforward way. For a photon to dislodge a photoelectron, it must have sufficient energy, by itself, to supply to the electron to overcome its attraction to the metal. Although increasing the intensity of the light does increase the total energy of the light, it does not increase the energy of an individual photon. Therefore, if the frequency of the light is too low, the photon energy is too low to eject an electron. Referring back to the analogy, we can say that a single bowling bowl can accomplish what many ping-pong balls cannot, and a single high frequency photon can accomplish what many low frequency photons cannot. The important conclusion for our purposes is that light energy is quantized into packets of energy. The amount of energy in each photon is given by Einsteinís equation,
where h is a constant called Planckís constant.
|
|||||||
Home  Atoms Atoms  Quantum Energy Levels In Atoms Quantum Energy Levels In Atoms  The Photoelectric Effect The Photoelectric Effect |
|||||||
Last Update: 2011-02-16